TLDR
- Sarepta Therapeutics (SRPT) stock jumped 40% in premarket trading after FDA allowed the company to resume shipping Elevidys therapy
- The FDA had requested a voluntary pause on shipments following safety concerns after patient deaths linked to the gene therapy
- Elevidys treats ambulatory patients with Duchenne muscular dystrophy and accounts for the majority of Sarepta’s revenue
- Stock had fallen 90% over the past 12 months before this news, with analyst sentiment turning negative
- Company needs strong Elevidys sales to manage $1.5 billion convertible debt due in 2027
Sarepta Therapeutics shares rocketed higher Tuesday morning after the FDA reversed its request for a voluntary shipping pause on the company’s gene therapy Elevidys. The stock jumped as much as 48% in early trading before settling around 35% higher at $18.75 in premarket action.
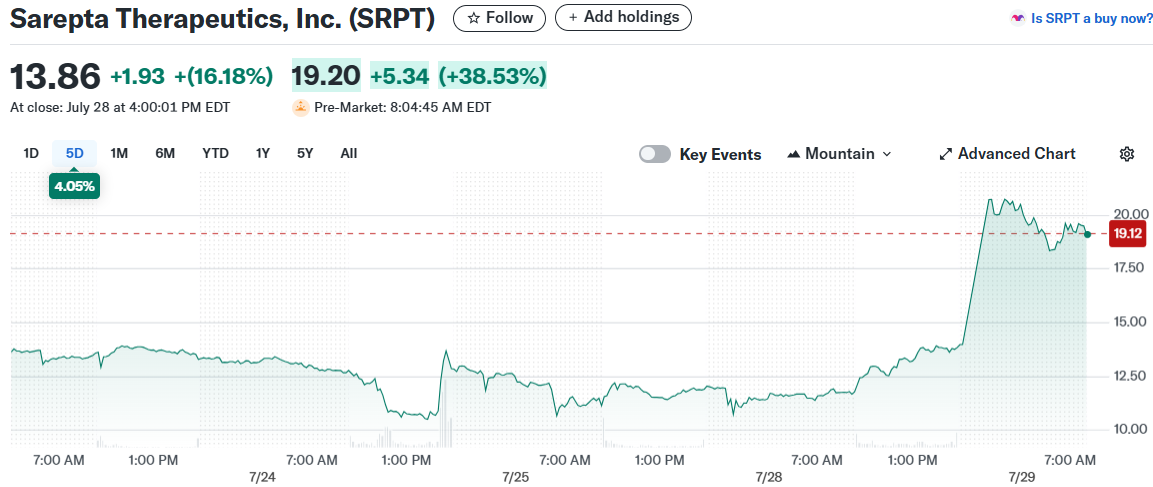
The FDA’s decision removes a major overhang that had crushed the biotech stock over recent months. Sarepta can now resume shipping Elevidys for treatment of ambulatory patients with Duchenne muscular dystrophy immediately.
The gene therapy represents the bulk of Sarepta’s revenue stream. Without it, the company faced serious financial pressure given its $1.5 billion convertible debt coming due in 2027.
CEO Doug Ingram expressed relief at the FDA’s quick review process. “We are very pleased that FDA chose to rapidly and comprehensively complete that review and to recommend that we remove our voluntary pause,” he said in Monday’s statement.
The shipping halt came after safety concerns emerged earlier this year. Two teenage boys with DMD who could no longer walk died from acute liver failure after receiving Elevidys treatments.
A third patient death involved a man taking a different gene therapy. Over the weekend, reports surfaced about another young boy’s death, but Sarepta clarified this was unrelated to Elevidys.
The FDA will continue investigating the patient deaths while shipments resume. The latest fatality involved a 51-year-old patient in a separate clinical trial for a different muscular dystrophy drug.
An 8-year-old boy in Brazil also died on June 7 from complications with a different therapy. Two confirmed Elevidys patient deaths have been reported this year.
Analyst Caution Remains High
Wall Street analysts remain divided on Sarepta’s prospects despite the positive FDA news. Cantor Fitzgerald’s Kristen Kluska noted that while the worst-case scenario is now off the table, “safety concerns keep us on the sidelines.”
Kluska maintains a Neutral rating with a $14 price target. The analyst community has grown increasingly skeptical of Sarepta over recent months.
Currently only 21% of the 29 analysts covering the stock rate it a Buy. About 59% assign Hold ratings while 21% recommend selling shares.
This marks a dramatic shift from April when 84% of analysts recommended the stock to clients. The European Medicines Agency panel’s recent recommendation to reject Elevidys added to the negative sentiment last week.
BMO Capital Markets analyst Kostas Biliouris bucked the trend by raising his price target to $50 from $25. He cited improving risk-reward dynamics while maintaining a Market Perform rating.
Leerink Partners analyst Joseph Schwartz acknowledged the positive development for patients but expressed caution. “We remain on the sidelines until we gain better visibility into how demand trends,” he wrote to clients.
Financial Pressure Mounts
The company’s debt situation adds urgency to getting Elevidys back on track. Sarepta needs consistent revenue from the therapy to handle its convertible debt obligations in 2027.
Jefferies analyst Andrew Tsai, one of the few maintaining a Buy rating, focused on the sales outlook. His firm expects Elevidys to remain on the market despite the safety concerns.
Tsai suggested that if Sarepta’s internal projections show the franchise generating at least $1.4 billion annually through 2027, investors might gain confidence in the company’s debt repayment ability. The stock had lost 90% of its value year-to-date before Tuesday’s surge.
Hospital systems had begun pausing dosing when Sarepta initially went against FDA wishes earlier in the process. This created additional uncertainty around future demand patterns for the treatment.
The FDA has now cleared the path for resumed shipments, giving Sarepta a chance to rebuild momentum with its cornerstone therapy.






