TLDR
- Plus Therapeutics (PSTV) stock surged 60% in premarket trading after securing a national agreement with UnitedHealthcare
- The agreement provides coverage for CNSide® test to over 51 million people across the United States, effective September 15, 2025
- H.C. Wainwright maintained a Buy rating with $3.00 price target, citing expanded reimbursement potential
- CNSide® test shows high sensitivity (92%) and specificity (95%) while influencing treatment decisions in 90% of cases
- Over 11,000 CNSide® tests have been performed at more than 120 U.S. cancer institutions since 2020
Plus Therapeutics stock jumped 60% in premarket trading Thursday following news of a breakthrough insurance agreement. The company secured national coverage from UnitedHealthcare for its CNSide® Cerebrospinal Fluid Tumor Cell Enumeration test.
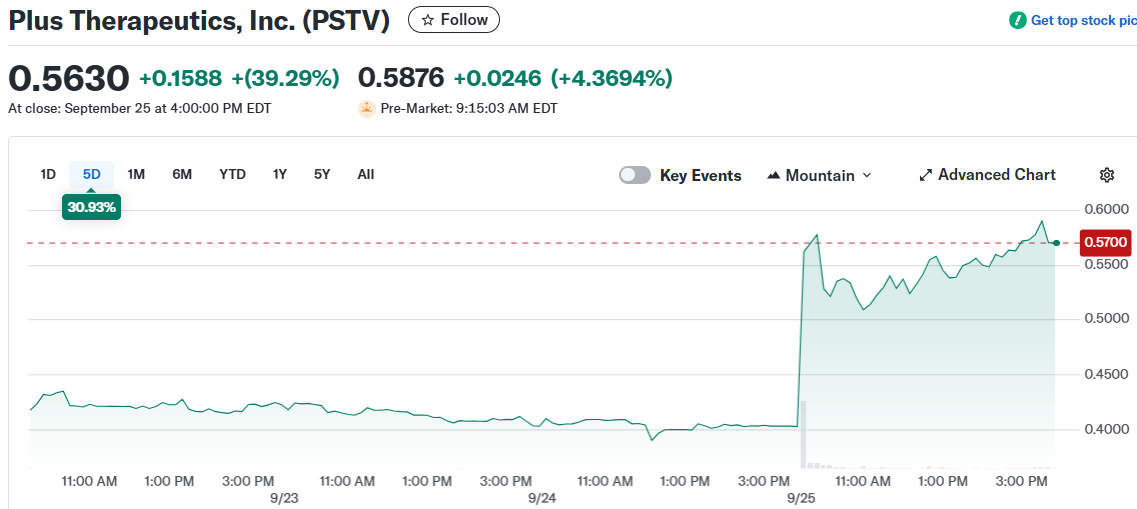
The agreement took effect September 15, 2025, and covers over 51 million people throughout the United States. This represents a major expansion in the test’s accessibility and revenue potential for the company.
H.C. Wainwright analyst Sean Lee maintained his Buy rating on the stock with a $3.00 price target. The analyst pointed to the UnitedHealthcare coverage as a key factor driving his positive outlook.
The CNSide® test helps doctors quickly diagnose and monitor treatment for patients with leptomeningeal metastases. This condition occurs when cancer spreads to the thin layers of tissue covering the brain and spinal cord.
Plus Therapeutics offers the test through CNSide Diagnostics, a wholly-owned subsidiary. The company has positioned this as an exclusive testing service for healthcare professionals across the country.
Clinical Performance Data
The test has shown strong performance metrics in clinical settings. It delivers 92% sensitivity and 95% specificity in detecting cancer cells in cerebrospinal fluid.
The test influences treatment decisions in 90% of cases where it’s used. This high impact rate makes it a valuable tool for oncologists treating complex cases.
Since 2020, medical professionals have performed more than 11,000 CNSide® tests. The test is now available at over 120 U.S. cancer institutions.
The company says the test has demonstrated superior clinical utility compared to standard care methods. This claim is backed by nine peer-reviewed publications and results from the FORESEE clinical trial.
Recent CLIA certification has opened doors for broader national availability. Combined with the UnitedHealthcare coverage, this creates a clear path for revenue growth.
Upcoming Catalysts
Sean Lee expects the upcoming Society for Neuro-Oncology annual meeting to provide additional positive momentum. The company plans to present updates from its ReSPECT-LM study during the conference.
Preliminary results from this study have shown promising response rates and favorable survival outcomes. These early findings suggest strong potential for the company’s product pipeline.
The analyst’s $3.00 price target reflects confidence in the company’s long-term revenue prospects. This valuation is supported by a risk-adjusted net present value analysis of the company’s innovative products.
The UnitedHealthcare agreement represents the largest insurance coverage deal in the company’s history. It provides access to a patient population that was previously difficult to reach through smaller regional agreements.
Plus Therapeutics now has a clear pathway to scale its CNSide® testing business nationwide. The September 15 effective date means revenue from this agreement should appear in the company’s fourth-quarter results.






