TLDR
- uniQure (QURE) stock surged over 220% in the past week following positive Phase 1/2 study results for AMT-130 gene therapy in Huntington’s disease
- The high-dose cohort showed a statistically significant 75% slowing of disease progression at 36 months compared to control data
- Secondary endpoints included 60% slowing in total functional capacity decline and neurodegeneration biomarkers remaining below baseline
- Company plans pre-BLA meeting with FDA in Q4 2025, targeting BLA filing in Q1 2026 under accelerated approval pathway
- Analysts raised price targets with Goldman Sachs modeling potential peak global sales of $2.5 billion
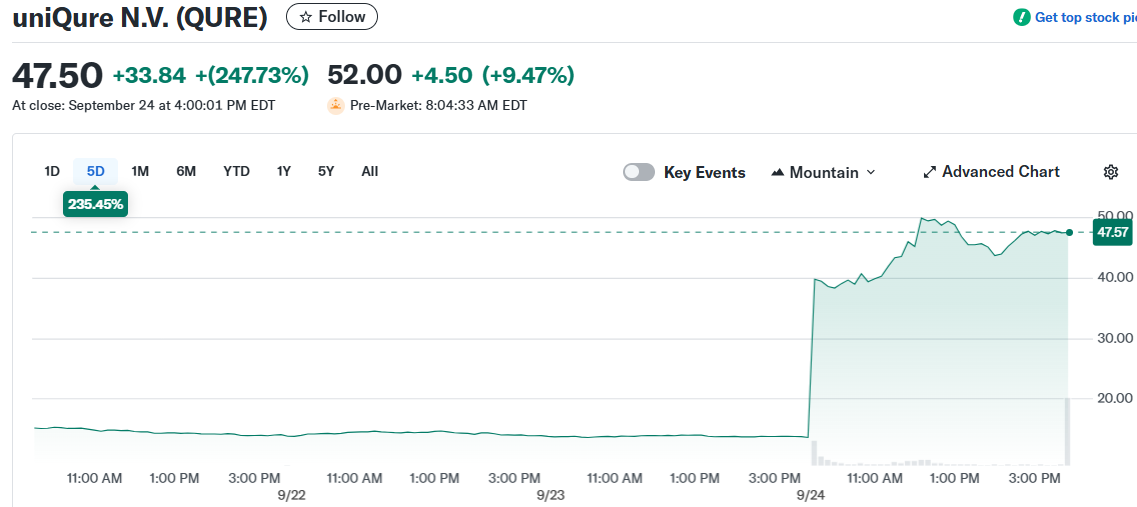
uniQure stock has experienced a dramatic rally this week, climbing over 220% following the release of positive clinical trial data. The biotech company announced topline results from its pivotal Phase 1/2 study of AMT-130 gene therapy for Huntington’s disease.
The stock approached its 52-week high of $51.21 as investors reacted to the clinical milestone. Trading volume has increased substantially from the average of 2.2 million shares.
The study focused on patients who received the high-dose treatment in the first two cohorts. These results will form the primary efficacy analysis for the company’s regulatory filing next year.
AMT-130 demonstrated a statistically significant 75% mean slowing of disease progression. This was measured using the composite Unified Huntington’s Disease Rating Scale compared to propensity score-matched natural history data at 36 months.
The therapy also showed a 60% slowing of disease progression in total functional capacity measures. This represents a secondary endpoint that supports the primary findings.
Biomarker Data Shows Promise
Cerebrospinal fluid neurofilament light protein levels remained below baseline throughout the study period. This biomarker indicates neurodegeneration and its stability suggests the therapy may be protecting brain tissue.
The safety profile remained consistent with previous data releases. No new treatment-related serious adverse events have been observed since December 2022.
The company plans to engage with the FDA in a pre-BLA meeting during the fourth quarter of 2025. This discussion will focus on the regulatory pathway for approval.
uniQure aims to file its Biologics License Application in the first quarter of 2026. The company will pursue an accelerated approval pathway based on the current data.
Analyst Response and Market Opportunity
Goldman Sachs maintained its Neutral rating with a $56 price target following the data release. The firm models potential peak global sales of $2.5 billion for AMT-130.
Their analysis assumes a 90% probability of success and approximately 25% cumulative penetration into the diagnosed patient population. An estimated 20,000 symptomatic patients are diagnosed in the United States.
Leerink Partners raised its price target to $68 from $48 while maintaining an Outperform rating. The firm cited the clinical results as particularly strong.
Stifel increased its price target to $65 from $30. The firm highlighted a potential U.S. market opportunity exceeding $1 billion in sales by 2031.
TD Cowen reiterated its Buy rating after the study met its primary endpoint. The firm noted the statistical significance of the disease progression slowing.
The company also announced a $200 million public offering of ordinary shares and pre-funded warrants. Leerink Partners, Stifel, Van Lanschot Kempen, and Guggenheim Securities are serving as bookrunning managers for the offering.
Stay Ahead of the Market with Benzinga Pro!
Want to trade like a pro? Benzinga Pro gives you the edge you need in today's fast-paced markets. Get real-time news, exclusive insights, and powerful tools trusted by professional traders:
- Breaking market-moving stories before they hit mainstream media
- Live audio squawk for hands-free market updates
- Advanced stock scanner to spot promising trades
- Expert trade ideas and on-demand support