TLDR
- Kenvue shares fell 7.5% Monday after Trump linked Tylenol to autism at White House press conference
- Stock bounced back 6% in premarket trading Tuesday as analysts cited lack of new scientific evidence
- Company disputed any link between acetaminophen and autism, warning against maternal health risks
- FDA will update labels but stressed no causal relationship has been established
- Analysts see limited lawsuit risk but potential impact on consumer behavior from negative headlines
Kenvue shares jumped 6% in premarket trading Tuesday, recovering from Monday’s sharp selloff. The rebound came after President Trump made unfounded claims linking Tylenol to autism during a White House news conference.
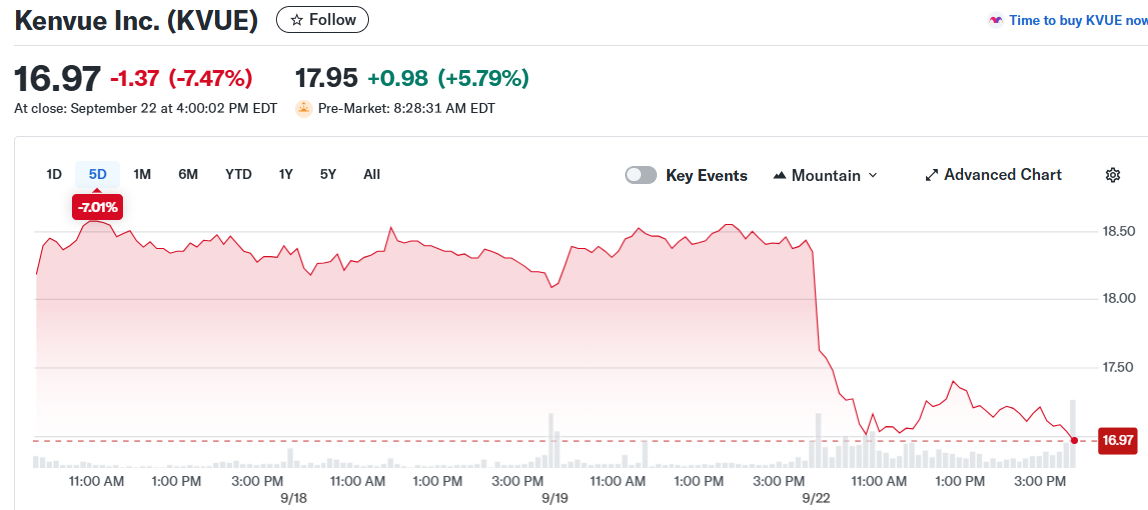
The New Jersey-based consumer health company saw its stock tumble 7.5% Monday. Shares hit an all-time low as Trump repeatedly warned pregnant women and mothers against using the popular pain medication.
Trump instructed pregnant women “Don’t take Tylenol” around a dozen times during Monday’s conference. He also urged mothers not to give the drug to their infants.
The medication is known by its generic name acetaminophen in the U.S. It goes by paracetamol in most other countries worldwide.
Kenvue was spun off from Johnson & Johnson in 2023. The company makes other household brands including Band-Aids and Listerine.
Market Response and Analyst Views
Citi Investment Research analyst Filippo Falorni sees limited risk of new lawsuits. However, he thinks “there could be risk to Tylenol consumption given the negative headlines.”
The company has already fought hundreds of lawsuits related to alleged autism ties. Most of these cases have been dismissed by courts.
Canaccord Genuity analyst Susan Anderson noted the current risk centers around public opinion. She pointed to potential brand damage from the negative publicity.
Analysts anticipated Tuesday’s positive stock reaction. They cited the absence of new scientific evidence supporting Trump’s claims.
The announcement appeared to rely on existing studies rather than new research. This comes as Health Secretary Robert F. Kennedy Jr. advances health initiatives targeting perceived autism causes.
Company and Regulatory Response
Kenvue disputed any link between acetaminophen and autism this week. The company warned that avoiding Tylenol could put pregnant mothers at risk.
Without the medication, mothers might face dangerous choices. They could either suffer through fevers or use riskier pain relief alternatives.
The Food and Drug Administration said it will update Tylenol labels. The changes will reflect evidence of possible links to neurological conditions like autism and ADHD.
However, the FDA stressed that no causal relationship has been established. The label updates are precautionary measures based on existing research.
A World Health Organization spokesperson addressed the claims Tuesday. They said evidence linking paracetamol use in pregnancy to autism remains inconsistent.
The WHO also emphasized that life-saving vaccines should not be questioned. This countered Trump’s broader claims about vaccine safety.
The European Medicines Agency confirmed no new evidence exists. They said current guidelines for the medication don’t need changes.
Shares of Kenvue climbed nearly 5% in early premarket trading. The stock has regained most of Monday’s losses as markets opened Tuesday.
Stay Ahead of the Market with Benzinga Pro!
Want to trade like a pro? Benzinga Pro gives you the edge you need in today's fast-paced markets. Get real-time news, exclusive insights, and powerful tools trusted by professional traders:
- Breaking market-moving stories before they hit mainstream media
- Live audio squawk for hands-free market updates
- Advanced stock scanner to spot promising trades
- Expert trade ideas and on-demand support



