TLDR
- Novo Nordisk stock jumped 5.7% in Copenhagen and 4.1% in premarket US trading after FDA approved Wegovy for liver disease treatment
- Wegovy becomes first GLP-1 drug approved for MASH (metabolic dysfunction-associated steatohepatitis), affecting 5% of US adults
- This approval opens access to a potential $30 billion market opportunity for Novo Nordisk
- The company faces increased competition from Eli Lilly and cheaper telehealth alternatives like Hims & Hers
- BMO analyst maintains Market Perform rating with $50 target price, citing potential momentum shift
Novo Nordisk shares climbed Monday morning following news that regulators approved its weight-loss drug Wegovy for treating a serious liver condition. The Danish drugmaker announced late Friday that the Food and Drug Administration cleared the medication for a new use.
WATCH: Shares in Novo Nordisk rose after it got US approval for its weight-loss drug Wegovy to treat a serious liver condition, positive news for the drugmaker that lost more than one-third of its market value in recent weeks https://t.co/rPN5MWC8RV pic.twitter.com/hkTRr57sLJ
— Reuters Business (@ReutersBiz) August 18, 2025
The FDA approved Wegovy to treat noncirrhotic metabolic dysfunction-associated steatohepatitis, known as MASH. This condition affects adults with liver fibrosis and involves fat buildup, inflammation, and damage in the liver.
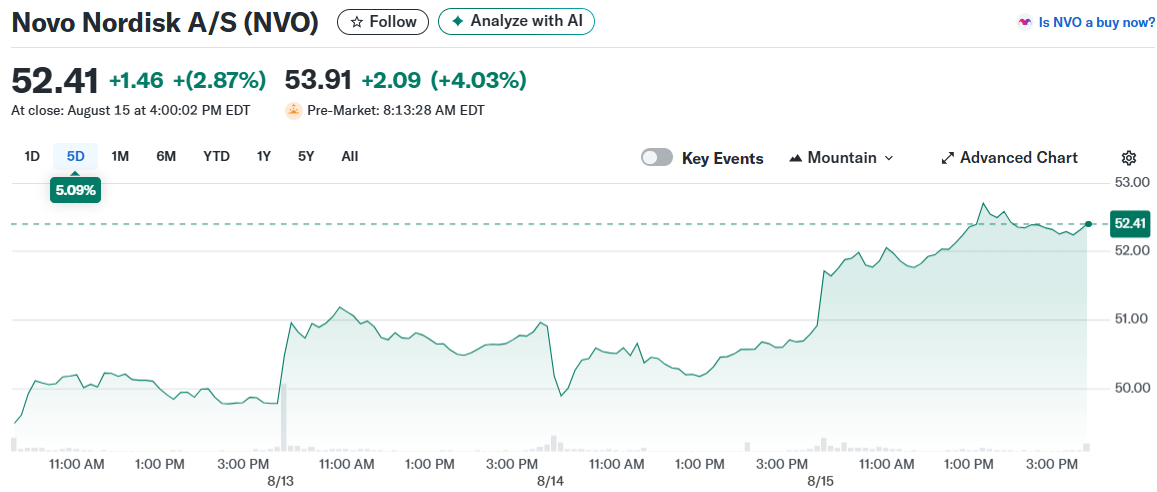
Novo Nordisk stock rose 5.7% in Copenhagen trading. The company’s American depositary receipts gained 4.1% to $53.97 in premarket trading. Competitor Eli Lilly shares moved 1.1% lower during the same period.
The approval makes Wegovy the first GLP-1 treatment cleared for MASH treatment. Chief Scientific Officer Martin Holst Lange called the drug “uniquely positioned” in this new market.
MASH affects approximately 5% of US adults according to the American Liver Foundation. This patient population represents a substantial market opportunity for Novo Nordisk.
The company manufactures semaglutide, which it markets as Wegovy for weight loss and Ozempic for diabetes treatment. Both drugs belong to the GLP-1 class, which mimics gut hormones that control blood sugar and reduce appetite.
Competitive Landscape Changes
The GLP-1 market has experienced rapid growth over recent years. Novo Nordisk initially dominated this space but now faces increased competition from multiple sources.
Eli Lilly has emerged as a major rival with its own GLP-1 products. Telehealth companies like Hims & Hers also offer cheaper versions of similar medications.
This competition has pressured Novo Nordisk’s market position. The company has reduced guidance multiple times this year as competitors gained ground.
Analyst Perspective
BMO analyst Evan Seigerman views the MASH approval as potentially important for Novo Nordisk’s momentum. He noted the company faced challenges earlier this year from competition and guidance cuts.
Seigerman maintains a Market Perform rating on the stock with a $50 target price. He believes the approval could help shift investor sentiment after a difficult period.
The approval opens access to what analysts estimate could be a $30 billion market opportunity. This represents a major expansion beyond Novo’s current weight-loss and diabetes markets.
Clinical trials showed Wegovy’s effectiveness in treating MASH patients. The drug demonstrated benefits for liver health in addition to its established weight-loss effects.
Novo Nordisk’s stock has declined 38.37% year-to-date despite Monday’s gains. The company trades with an average daily volume of 14.5 million shares and maintains a market capitalization of $226.9 billion.